How Many Electrons Does Carbon Have? Unlock the Mystery!
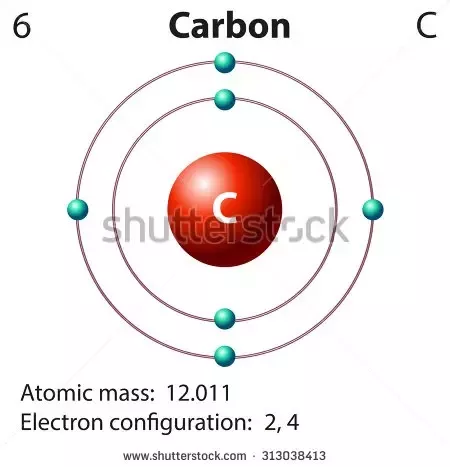
Carbon has 6 electrons. Carbon, a chemical element with atomic number 6, possesses 6 electrons in its atomic structure.
This makes it a stable element with a balanced electron configuration. Carbon is widely known for its ability to form strong covalent bonds with other atoms, making it the building block of organic compounds and the foundation of life on Earth.
Its unique electron arrangement allows carbon to exhibit diverse chemical properties, leading to its involvement in various biological and industrial processes. Understanding the electron count of carbon is essential in comprehending its role in chemical reactions and its significance in numerous scientific fields, including chemistry, biology, and materials science.
The Basics Of Carbon
Carbon, a fundamental element, has six electrons in its outer shell. This configuration makes carbon versatile for forming various compounds.
Carbon’s Place In The Periodic Table
How Many Electrons Does Carbon Have? Carbon is a chemical element that has the symbol C and atomic number 6. It is a nonmetal, tetravalent element, which means that it can form four covalent bonds with other atoms. Carbon is an essential element for life on Earth, and it is the basis of organic chemistry, the study of carbon-containing compounds. Carbon belongs to Group 14 of the periodic table, also known as the carbon group. This group consists of carbon, silicon, germanium, tin, and lead. These elements share similar properties, such as having four valence electrons and forming covalent bonds. Carbon is the second most abundant element in the human body, after oxygen.
Atomic Structure Fundamentals
The atomic structure of carbon is fundamental to understanding its chemical properties. Carbon has six electrons, with two electrons in the first energy level and four electrons in the second energy level. The four valence electrons of carbon are located in the outermost energy level, which is why carbon can form four covalent bonds with other atoms. The electron configuration of carbon is 1s2 2s2 2p2. This means that the first energy level contains two electrons in the 1s orbital, and the second energy level contains two electrons in the 2s orbital and two electrons in the 2p orbital.
The 2p orbital contains three suborbitals, which can hold a total of six electrons. Carbon has two stable isotopes, carbon-12 and carbon-13. Carbon-12 is the most abundant isotope, accounting for 98.93% of all carbon atoms. Carbon-13 makes up the remaining 1.07% of carbon atoms. In conclusion, understanding the basics of carbon, including its place in the periodic table and atomic structure fundamentals, is essential to understanding its chemical properties and its importance in organic chemistry and life on Earth.
Carbon’s Electron Configuration
Quantum Mechanics And Electron Arrangement
Quantum mechanics explains electron arrangement in carbon.
Energy Levels And Electron Shells
Carbon has 6 electrons in 2 energy levels and 4 valence electrons.
Visualizing Carbon’s Electrons
Carbon has six electrons in its outer shell, giving it a total of six electrons. Understanding carbon’s electron configuration is crucial in chemistry and biology. By visualizing carbon’s electrons, we can grasp its bonding properties and role in various compounds.
Understanding the electron configuration of an atom is crucial in comprehending its chemical behavior. In the case of carbon, an element that is the basis of life on Earth, visualizing its electrons can provide valuable insights. Two commonly used methods for visualizing carbon’s electrons are orbital diagrams and electron dot structures.
Orbital Diagrams Explained
Orbital diagrams are a visual representation of the arrangement of electrons within an atom’s orbitals. These diagrams use boxes to represent orbitals, with arrows indicating the electrons’ spins. For carbon, which has an atomic number of 6, the orbital diagram illustrates the arrangement of its six electrons in the 1s, 2s, and 2p orbitals.
In the 1s orbital, there are two electrons, each represented by an upward or downward arrow. The 2s orbital also holds two electrons, while the remaining two electrons occupy the 2p orbital. The 2p orbital consists of three separate orbitals, each capable of holding a maximum of two electrons. Therefore, carbon’s 2p orbital contains one unpaired electron in each of its three p orbitals.
Electron Dot Structures
Electron dot structures, also known as Lewis dot structures, provide a simplified representation of an atom’s valence electrons. In the case of carbon, its electron dot structure showcases the four valence electrons present in its outermost energy level. These electrons are denoted by dots surrounding the atomic symbol.
Carbon’s electron dot structure features two dots on each side of the atomic symbol, depicting the two unpaired electrons in the 2p orbital. This arrangement allows carbon to form multiple covalent bonds with other atoms, enabling the vast array of organic compounds found in nature.
Visualizing carbon’s electrons through orbital diagrams and electron dot structures aids in understanding its chemical reactivity and bonding capabilities. By examining the arrangement of electrons, scientists can predict how carbon interacts with other elements and forms the intricate molecular structures essential for life.
Credit: www.quora.com
Carbon’s Role In Organic Compounds
Carbon is a crucial element in the organic world. It is the fourth most abundant element in the universe and the second most abundant element in the human body. Carbon forms the backbone of all living organisms and is involved in the formation of many essential molecules. Understanding the bonding capabilities and versatility of carbon in chemistry is essential in understanding the role it plays in organic compounds.
Bonding Capabilities Of Carbon
Carbon has four valence electrons, which allows it to form four covalent bonds with other atoms. This bonding capability is the basis of organic chemistry. Carbon can form single, double, or triple bonds with other carbon atoms or other elements such as hydrogen, oxygen, and nitrogen. The ability to form multiple bonds allows for the creation of complex structures, making carbon the building block of life.
The Versatility Of Carbon In Chemistry
The versatility of carbon lies in its ability to form a wide range of compounds due to its bonding capabilities. Carbon can form linear, branched, and cyclic molecules, which can vary in size and complexity. This versatility allows for the creation of a vast array of organic compounds, from simple sugars to complex proteins and DNA. Carbon’s ability to form stable covalent bonds is also the reason why organic compounds tend to be stable under normal conditions, unlike inorganic compounds that can easily break down.
| Examples of Organic Compounds |
|---|
| Carbohydrates |
| Lipids |
| Proteins |
| Nucleic Acids |
| Enzymes |
- Carbon forms the backbone of all living organisms.
- Carbon can form single, double, or triple bonds with other atoms.
- Carbon’s versatility allows for the creation of a vast array of organic compounds.
- Organic compounds tend to be stable under normal conditions.
- Examples of organic compounds include carbohydrates, lipids, proteins, and nucleic acids.
Carbon’s unique bonding capabilities and versatility in chemistry make it an essential element in the organic world. Understanding the role of carbon in organic compounds is crucial in fields such as biology, medicine, and materials science.
Isotopes Of Carbon
Variations In Carbon’s Atomic Structure
Carbon, a fundamental element in the periodic table, typically contains six protons and six electrons. However, its atomic structure can vary due to the presence of different numbers of neutrons, resulting in isotopes. These isotopes have the same number of protons but differ in their neutron count, affecting their atomic mass and stability.
Stability And Radioactivity Of Carbon Isotopes
Carbon isotopes exhibit varying levels of stability and radioactivity based on their neutron count. For instance, Carbon-12, the most abundant and stable isotope, is non-radioactive. Conversely, Carbon-14, with two extra neutrons, is radioactive and commonly used in carbon dating to determine the age of organic materials.
Electrons And Chemical Reactivity
Carbon, a chemical element, has six electrons. Understanding the electron configuration of carbon is crucial in comprehending its chemical reactivity and its role in various compounds and reactions.
Understanding the role of electrons in chemical reactivity is essential for comprehending the behavior of different elements and compounds. In the case of carbon, its electron count greatly influences its reactivity and ability to form chemical bonds. Let’s explore how this electron count affects carbon’s valence electrons and chemical bonds.
How Electron Count Influences Reactivity
The number of electrons in an atom determines its reactivity. Carbon, with an atomic number of 6, has 6 electrons in total. These electrons are distributed in different energy levels or shells around the nucleus. The outermost shell, called the valence shell, plays a crucial role in determining the reactivity of carbon.
Carbon has four valence electrons in its outermost shell. This means that carbon can form up to four chemical bonds with other atoms. These bonds can be either single, double, or triple bonds, depending on the number of electrons shared between carbon and the other atom.
Carbon’s Valence Electrons And Chemical Bonds
The four valence electrons of carbon allow it to form a wide range of chemical bonds, making it the building block for countless organic compounds. Carbon can bond with other carbon atoms, forming long chains or rings, giving rise to the vast diversity of organic molecules found in nature.
Additionally, carbon can form bonds with other elements such as hydrogen, oxygen, nitrogen, and many others. These bonds are essential for the formation of functional groups and the stability of various compounds.
It is worth noting that the presence of double or triple bonds in carbon compounds can significantly influence their reactivity and properties. These multiple bonds provide additional strength and stability to the molecules, allowing them to participate in various chemical reactions.
In conclusion, the electron count of carbon, specifically its four valence electrons, plays a crucial role in determining its reactivity and ability to form chemical bonds. Understanding the behavior of carbon’s valence electrons is fundamental to grasp the intricate chemistry of organic compounds and their diverse applications.
Carbon In Everyday Life
Carbon, a vital element in everyday life, contains 6 electrons. It plays a crucial role in forming the building blocks of life and is found in various forms, from diamonds to the graphite in pencils. Understanding the electron configuration of carbon is fundamental to comprehend its significance in the natural world.
The Presence Of Carbon In Materials
Carbon is widely present in various materials we encounter daily.
From plastics to fabrics, carbon is a fundamental component.
Even in electronics, carbon plays a crucial role.
Carbon’s Ecological Footprint
Carbon has a significant impact on the environment.
Its emissions contribute to climate change.
Reducing carbon footprint is essential for sustainability.
Advancements In Carbon Research
Advancements in Carbon Research: Carbon research has seen remarkable progress in recent years, leading to groundbreaking discoveries.
Nanotechnology And Carbon Structures
Nanotechnology explores the manipulation of matter at the atomic and molecular levels. Carbon nanostructures like nanotubes and fullerenes have revolutionized various industries.
Future Applications Of Carbon In Science
The versatility of carbon opens doors to exciting possibilities in fields like medicine and renewable energy. Graphene, a one-atom-thick carbon material, shows promise in electronics and aerospace.

Credit: www.britannica.com
Frequently Asked Questions
How Many Electrons Does Carbon Have?
Carbon has 6 electrons, with 2 in the first shell and 4 in the second shell. This configuration allows it to form strong covalent bonds with other atoms, making it an essential building block of life.
What Is The Significance Of Carbon’s Electron Configuration?
The electron configuration of carbon allows it to form a wide variety of compounds, including the complex molecules essential for life. This versatility makes carbon a cornerstone of organic chemistry and the basis for all known life forms.
How Does The Number Of Electrons Affect Carbon’s Reactivity?
With 4 electrons in its outer shell, carbon can form strong covalent bonds with other elements, resulting in a wide range of compounds. This ability to form diverse molecular structures contributes to the vast array of carbon-based materials and organic compounds found in nature.
Conclusion
Carbon has six electrons, with two in its inner shell and four in its outer shell. This configuration allows carbon to form a wide variety of compounds, making it essential for life on Earth. Understanding carbon’s electron arrangement is fundamental to comprehending its role in chemistry and biology.





