How Many Electrons Does Oxygen Have? 8 Mystery Revealed!

How many electrons does oxygen have? It possesses 8 electrons in its atomic structure, rendering it a crucial element.
Oxygen, an essential element on the periodic table, possesses 8 electrons in its atomic structure. This key characteristic defines its chemical behavior and role in various biological and environmental processes. With a symbol “O” and atomic number 8, oxygen plays a vital role in sustaining life through processes such as respiration and combustion.
Its electron configuration contributes to its ability to form bonds with other elements, creating diverse compounds essential for life on Earth. Understanding the electron count of oxygen is fundamental in comprehending its significance in both natural and synthetic chemical reactions.
Credit: www.quora.com
Peering Into The Atomic Realm
Oxygen, a chemical element, has eight electrons in its atomic structure. Peering into the atomic realm reveals the precise arrangement of these electrons, which play a crucial role in oxygen’s chemical properties and interactions.
Peering Into the Atomic Realm: The world around us is composed of atoms, the building blocks of matter. How many electrons does oxygen have? Atoms are incredibly small, so small that they cannot be seen with the naked eye. However, with the use of powerful microscopes, scientists have been able to peer into the atomic realm and unravel the mysteries of the atomic structure.
In this article, we will explore the basics of atomic structure, focusing on the number of electrons oxygen has and their role in chemical identity. The Basics of Atomic Structure: An atom is made up of three subatomic particles: protons, neutrons, and electrons. How many electrons does oxygen have? Protons and neutrons are located in the nucleus, the center of the atom, while electrons orbit around the nucleus in shells or energy levels.
The number of protons in an atom determines its atomic number and the element it represents. For instance, oxygen has an atomic number of 8, which means it has 8 protons. Electrons: The Bearers of Chemical Identity: How many electrons does oxygen have? Electrons play a crucial role in determining the chemical properties of an element. The electrons in the outermost shell, also known as the valence shell, are responsible for the chemical behavior of an element. How many electrons does oxygen have? Oxygen has 8 electrons, 2 in the first shell and 6 in the second shell.
The valence shell of oxygen has 6 electrons, which makes it highly reactive and a key component in many chemical reactions. In conclusion, oxygen has 8 electrons, with 6 in the valence shell. How many electrons does oxygen have? These electrons play a vital role in determining the chemical properties of oxygen and its reactivity in chemical reactions. Understanding the basics of atomic structure and the role of electrons is crucial in the field of chemistry and provides a foundation for further scientific exploration.
Oxygen: Essential Element Of Life
The Role Of Oxygen In Nature
Oxygen plays a crucial role in sustaining life on Earth. From the air we breathe to the water we drink, oxygen is an essential component of the environment. It is a key element in the process of respiration for most living organisms, including humans, animals, and plants.
Oxygen’s Place In The Periodic Table
Oxygen, represented by the symbol O and atomic number 8, is a member of the chalcogen group on the periodic table. How many electrons does oxygen have? It is a non-metal that readily forms compounds with almost all other elements. In its diatomic form (O2), oxygen constitutes about 20% of the Earth’s atmosphere.
Counting Electrons
How Electrons Are Arranged
The arrangement of electrons within an atom plays a crucial role in determining its chemical properties. How many electrons does oxygen have? Electrons occupy different energy levels or orbitals around the nucleus. These energy levels are often referred to as shells, and each shell can accommodate a specific number of electrons. The arrangement of electrons follows the Aufbau principle, which states that electrons fill the lowest energy levels first before moving to higher ones.
How Many Electrons Does Oxygen Have: Methods To Determine Electron Count
Determining the number of electrons in an atom can be done using various methods. How many electrons does oxygen have? Here are a few commonly used techniques:
- Periodic Table: The periodic table provides valuable information about an element’s electron configuration. By referring to the periodic table, you can identify the position of the element and determine the number of electrons it possesses.
- Valence Electrons: Valence electrons are the outermost electrons in an atom’s electron configuration. For many elements, the number of valence electrons corresponds to their group number on the periodic table. This method is particularly useful when determining the electron count for elements in the s and p blocks.
- Electron Configuration: The electron configuration of an atom represents the specific arrangement of electrons within its orbitals. By understanding the rules governing electron configuration, such as the Pauli exclusion principle and Hund’s rule, you can determine the electron count for a given element.
Counting electrons is essential for understanding an atom’s chemical behavior and its ability to form bonds with other atoms. By utilizing these methods, scientists can accurately determine the electron count of different elements, including oxygen.
The Electron Configuration Of Oxygen
Understanding Energy Levels
Electrons in an atom are organized into energy levels. The first energy level can hold up to 2 electrons.
The second energy level can hold up to 8 electrons.
The third energy level can hold up to 18 electrons.
Oxygen has 8 electrons, so it fills the first two energy levels completely.
Orbitals And Oxygen: A Closer Look
Within each energy level, electrons are further organized into orbitals. Each orbital can hold a maximum of 2 electrons.
Oxygen’s electron configuration is 1s2 2s2 2p4, indicating the distribution of its 8 electrons.
Chemical Bonds And Oxygen
Oxygen forms chemical bonds through covalent bonding and ionic bonding.
Covalent Bonding In Oxygen
Oxygen shares electrons with other elements to form covalent bonds.
Oxygen’s Valency And Bond Formation
Oxygen has a valency of 2, allowing it to form bonds with other elements.
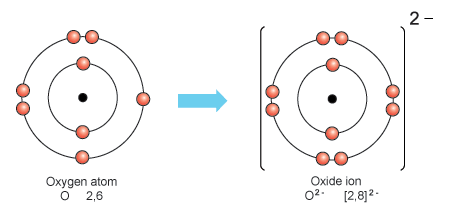
Oxygen Ions: Variations In Electron Count
Oxygen ions exhibit varying electron counts depending on their oxidation states. Oxygen typically has 8 electrons, but can gain or lose electrons to achieve stability. This characteristic of oxygen ions plays a crucial role in chemical reactions and biological processes.
Oxygen ions can have different electron counts.
Oxide Anions And Their Electron Gain
Oxide anions gain 2 electrons to form stable compounds.
The Formation Of Peroxides And Superoxides
Peroxides gain one more electron, superoxides gain two more. Oxygen ions can have various electron counts. Oxide anions gain 2 electrons. Peroxides gain 1 more, superoxides gain 2 more.
Isotopes Of Oxygen
Oxygen has 8 electrons, with 6 of them in the outer shell. Isotopes of oxygen vary in the number of neutrons, but all isotopes have 8 protons and 8 electrons.
Stable And Unstable Isotopes
Oxygen has stable and unstable isotopes, each with a unique number of electrons.
Electron Counts In Different Isotopes
Here is a breakdown of electron counts in various oxygen isotopes: | Isotope | Number of Electrons | | ————— | ——————– | | Oxygen-16 | 8 | | Oxygen-17 | 8 | | Oxygen-18 | 8 | Isotopes of Oxygen Oxygen has different isotopes, including stable and unstable variations. Stable and Unstable Isotopes Oxygen isotopes can be stable or unstable based on their electron count. Electron Counts in Different Isotopes – Oxygen-16 has 8 electrons. – Oxygen-17 has 8 electrons. – Oxygen-18 has 8 electrons.
Applications And Implications
Oxygen, with its six electrons, plays a crucial role in various applications and has significant implications in different fields. From industrial processes to its impact on biological systems, understanding the applications and implications of oxygen’s electrons is essential.
Oxygen In Industrial Processes
In industrial processes, oxygen’s electron configuration makes it a versatile element with numerous applications. It is widely used in:
- Combustion processes, where oxygen supports the burning of fuels, providing heat and energy.
- Oxidation reactions, where oxygen participates in chemical reactions that involve the transfer of electrons.
- Metallurgical processes, such as steelmaking, where oxygen is used to remove impurities and enhance the quality of the final product.
- Water treatment processes, where oxygen plays a role in disinfection and oxidation of contaminants.
Electron Impact On Biological Systems
The presence of oxygen and its electrons significantly impacts biological systems, including both living organisms and cellular processes. Some key implications include:
- Oxygen’s involvement in cellular respiration, where it accepts electrons during the electron transport chain, producing energy in the form of ATP.
- Reactive oxygen species (ROS) formation, which occurs when oxygen molecules gain or lose electrons, leading to oxidative stress and potential damage to cells and DNA.
- Oxygen’s role in the immune system, where it is utilized by immune cells to produce reactive oxygen species for defense against pathogens.
- Oxygen’s importance in wound healing processes, as it supports the growth and survival of cells involved in tissue repair.
Understanding the applications and implications of oxygen’s electron configuration provides valuable insights into its role in various industries and biological systems. From powering industrial processes to influencing cellular functions, the six electrons of oxygen contribute to its significance in the world around us.
Visualizing Electron Movement
Oxygen, a fundamental element in the periodic table, plays a crucial role in various chemical and biological processes. How many electrons does oxygen have? Understanding the movement of its electrons is essential in comprehending its behavior and reactivity.
Spectroscopy And Electron Transitions
Spectroscopy allows scientists to study the movement of electrons in oxygen by analyzing the interaction of matter with electromagnetic radiation. This technique provides valuable insights into the energy levels and transitions of oxygen’s electrons, aiding in the visualization of their movements within the atom.
Electron Microscopy And Oxygen
Electron microscopy offers a powerful tool for visualizing the arrangement and behavior of oxygen’s electrons at the atomic scale. This advanced imaging technique enables scientists to observe the precise distribution and movement of electrons within oxygen, contributing to a deeper understanding of its properties and interactions.
Teaching The Concept
Oxygen has eight electrons in its atomic structure. How many electrons does oxygen have? Teaching the concept of the number of electrons in oxygen is fundamental to understanding its chemical behavior and reactivity. This knowledge is essential in various scientific fields, from chemistry to biology and environmental science.
Teaching the Concept: Understanding the concept of electrons and their count is essential to comprehend the chemical and physical properties of elements. How many electrons does oxygen have? Oxygen, a crucial element for life, has eight electrons in its outer shell, which makes it highly reactive. However, teaching this concept can be challenging for educators. How many electrons does oxygen have? In this article, we will discuss some educational approaches to teach the concept of electron count, with a focus on interactive models that can help students grasp the concept better.
Educational Approaches to Electron Count: When it comes to teaching the concept of electron count, educators must use different educational approaches to cater to diverse learning styles. How many electrons does oxygen have? Some of the educational approaches that can be used are: 1. Visual Learning: Using visual aids such as diagrams, images, and videos can help students understand the concept of electron count better. How many electrons does oxygen have? Educators can use various software to create interactive models that show the movement of electrons. 2. Hands-on Learning: Practical experiments can help students understand the concept of electron count. How many electrons does oxygen have? For instance, educators can conduct experiments to demonstrate the transfer of electrons between atoms.
Interactive Models for Better Understanding: How many electrons does oxygen have? Interactive models are an effective way to teach the concept of electron count. These models allow students to manipulate and observe the movement of electrons. How many electrons does oxygen have? Some interactive models that can be used are: 1. Bohr Model: The Bohr model is a visual representation of an atom that shows the arrangement of electrons in different energy levels. How many electrons does oxygen have? Educators can use this model to explain the concept of electron count. 2. Lewis Dot Structure: The Lewis Dot Structure is another interactive model that shows the arrangement of electrons in an atom.
This model uses dots to represent electrons, making it easier for students to understand the concept of electron count. How many electrons does oxygen have? In conclusion, teaching the concept of electron count can be challenging, but with the right educational approaches and interactive models, educators can make it easier for students to understand. How many electrons does oxygen have? By using visual aids, hands-on learning, and interactive models, educators can cater to diverse learning styles and help students grasp the concept of electron count better.
.
Credit: socratic.org
Frequently Asked Questions
How Many Electrons Does Oxygen Have?
Oxygen has 8 electrons. It is an element with atomic number 8, which means it has 8 protons and 8 electrons in its neutral state. Electrons are negatively charged particles that orbit around the nucleus of an atom, and they play a crucial role in chemical reactions and bonding.
Conclusion
Oxygen has eight electrons. This element is critical for life and is involved in many chemical reactions in the body. Understanding its electron configuration is important for understanding its properties and behavior. By knowing the number of electrons, scientists can predict how oxygen will interact with other elements and molecules.
It is fascinating to think about how something as small as an electron can have such a big impact on our world.





