How Many Valence Electrons Does Oxygen Have: Unveiled!
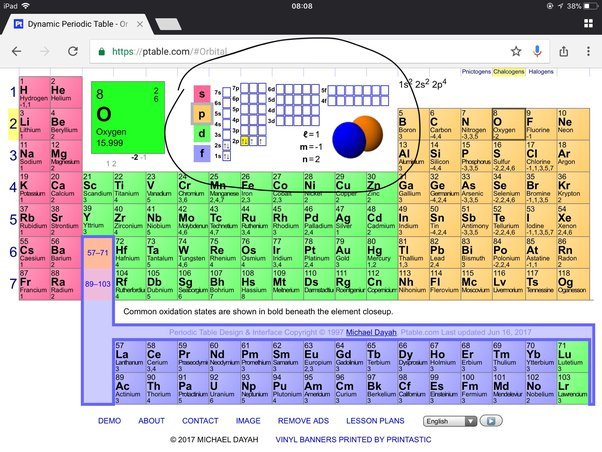
Oxygen has 6 valence electrons. Oxygen, with its atomic number of 8, belongs to Group 16 of the periodic table, also known as the oxygen family.
This group contains elements that typically have 6 valence electrons. Valence electrons are the outermost electrons in an atom and are responsible for an element’s chemical properties and reactivity. In the case of oxygen, its 6 valence electrons enable it to form stable compounds with other elements, making it an essential component of many biological and chemical processes.
Understanding the number of valence electrons in an atom is crucial for predicting its behavior in chemical reactions and understanding its role in various systems.

Credit: www.youtube.com
The Basics Of Valence Electrons
Oxygen has six valence electrons.
Electron Configuration
Oxygen’s electron configuration is 1s2 2s2 2p4.
Valence Shell Concept
The valence shell is the outermost shell of an atom.
Credit: socratic.org
Oxygen’s Place In The Periodic Table
How Many Valence Electrons Does Oxygen Have? Oxygen, situated in group 16 of the periodic table, has 6 valence electrons. These electrons play a crucial role in oxygen’s chemical reactivity and bonding properties, making it essential for various biological and industrial processes.
Oxygen is a non-metallic element with atomic number 8 and symbol O. It belongs to group 16 of the periodic table, also known as the chalcogens, which includes elements such as sulfur, selenium, and tellurium. Oxygen is the second most abundant element in the Earth’s atmosphere, making up 21% of it by volume. Its place in the periodic table plays a crucial role in determining its chemical behavior and properties.
Periodic Group And Its Significance
The periodic table is an essential tool used to organize the elements based on their atomic structure and chemical properties. Oxygen belongs to group 16, also known as the oxygen family, which is located on the right side of the periodic table. The elements in this group have six valence electrons, making them highly reactive. Oxygen, in particular, has a strong affinity for electrons, making it an excellent oxidizing agent. This property is essential for various biological and chemical processes.
Electronegativity And Reactivity
Oxygen has a high electronegativity, meaning it has a strong attraction for electrons. This property makes it highly reactive and capable of forming strong covalent bonds with other elements, especially metals. Oxygen’s reactivity and electronegativity play a vital role in the formation of the Earth’s atmosphere and its effects on the environment. Oxygen is also essential for various biological processes, such as respiration and metabolism.
In conclusion, Oxygen’s place in the periodic table is significant in determining its chemical behavior and properties. Its high electronegativity and reactivity make it an important element for various chemical and biological processes. Understanding oxygen’s role in the periodic table can help us better understand its effects on the environment and how it interacts with other elements.
Diving Into Oxygen’s Electron Arrangement
Understanding the electron arrangement of oxygen is crucial in comprehending its chemical behavior and reactivity. In this section, we will explore the atomic structure of oxygen, its energy levels, and the orbitals in which its valence electrons reside.
Atomic Structure
Oxygen, with the chemical symbol O and atomic number 8, has an atomic structure consisting of eight protons, eight neutrons, and eight electrons. These subatomic particles play a vital role in determining oxygen’s properties and interactions with other elements.
Energy Levels And Orbitals
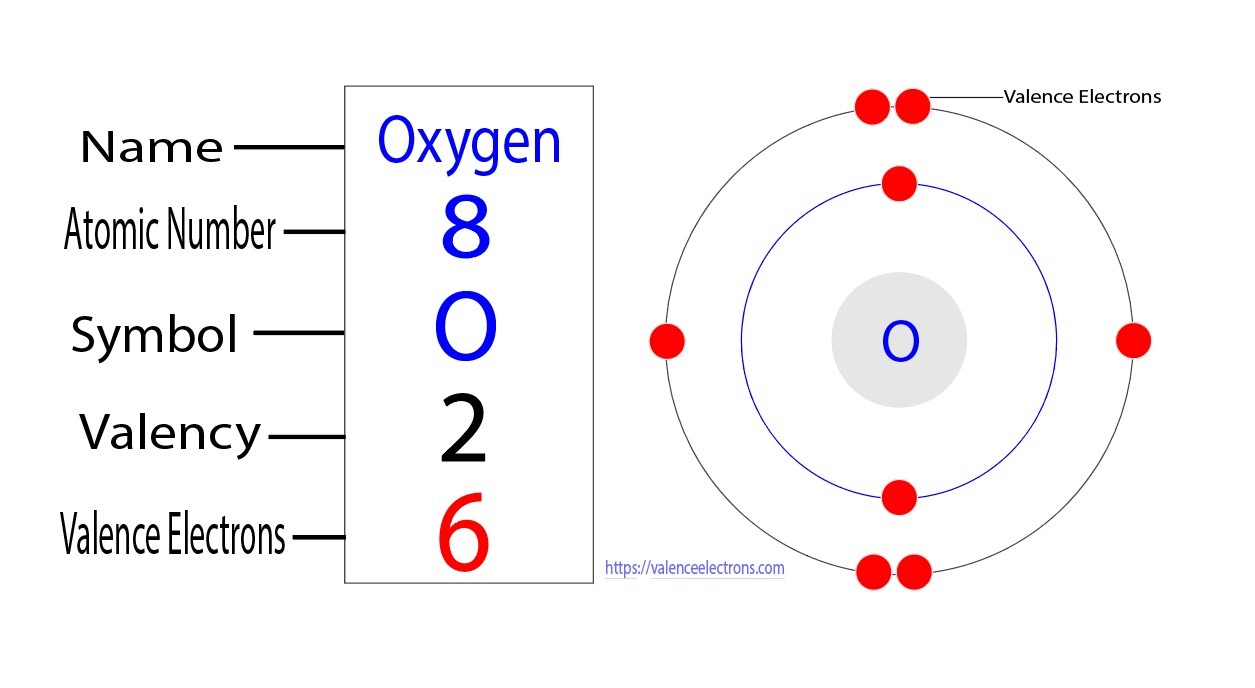
Oxygen’s electrons are organized into different energy levels or shells, each with a specific capacity to hold electrons. The first energy level can accommodate a maximum of two electrons, while the second energy level can hold up to eight electrons. In the case of oxygen, the first energy level contains two electrons, and the second energy level contains six electrons.
Within each energy level, there are different sublevels known as orbitals. These orbitals further divide the energy levels into regions where electrons are most likely to be found. Oxygen’s second energy level consists of two types of orbitals: s orbitals and p orbitals. The s orbitals can hold a maximum of two electrons, while the p orbitals can accommodate up to six electrons.
To visualize the electron arrangement of oxygen, we can represent it using the electron configuration notation. Oxygen’s electron configuration is 1s2 2s2 2p4, which indicates the distribution of its eight electrons across the energy levels and orbitals. The superscripts represent the number of electrons in each orbital.
In summary, oxygen has a total of eight valence electrons, which are the electrons in the outermost energy level. These valence electrons play a significant role in the chemical reactions and bonding behavior of oxygen with other elements.
Understanding the electron arrangement of oxygen provides a foundation for comprehending its chemical properties, reactions, and participation in various biological processes. Let’s explore further in the upcoming sections.
Counting Oxygen’s Valence Electrons
Oxygen, a chemical element, has 6 valence electrons. Understanding the count of valence electrons in oxygen is crucial for predicting its chemical behavior and interactions with other elements.
The Octet Rule
The octet rule states that atoms tend to gain, lose, or share electrons to achieve a stable configuration of 8 valence electrons. Oxygen has 6 valence electrons, so it needs 2 more electrons to complete its valence shell and attain stability.
Electron Dot Diagrams
An electron dot diagram, also known as a Lewis structure, represents the valence electrons of an atom as dots around the elemental symbol. For oxygen, each dot represents one valence electron. The electron dot diagram of oxygen consists of two paired dots on one side and two unpaired dots on the other side.
Valence Electrons Of Oxygen
To count the valence electrons of oxygen, we simply count the number of dots in its electron dot diagram, which gives us 6 valence electrons. However, we also need to consider the octet rule, which means oxygen requires 2 more electrons to complete its valence shell. Therefore, the total valence electrons of oxygen is 8, with 6 valence electrons represented by dots and 2 more needed to attain stability. Knowing the valence electrons of oxygen is crucial in predicting its chemical behavior and its ability to form compounds with other elements.
The Role Of Valence Electrons In Chemical Bonding
When it comes to understanding the behavior of atoms in chemical bonding, the concept of valence electrons plays a crucial role. Valence electrons are the electrons in the outermost energy level of an atom that are involved in the formation of chemical bonds with other atoms. The number of valence electrons an atom possesses determines its reactivity and its ability to form different types of chemical bonds.
Covalent Bonding
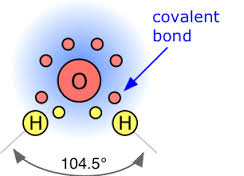
In covalent bonding, atoms share pairs of valence electrons to achieve a full outer energy level. This type of bonding occurs between nonmetal atoms and results in the formation of molecules. Covalent bonds are strong and are responsible for the stability of many compounds, including water and organic molecules.
Ionic Bonding
In ionic bonding, one atom transfers one or more valence electrons to another atom, resulting in the formation of positively and negatively charged ions that are held together by electrostatic forces. This type of bonding usually occurs between a metal and a nonmetal, leading to the formation of ionic compounds such as table salt and magnesium oxide.
Oxygen In Compounds
Oxygen has 6 valence electrons, making it highly reactive in forming compounds. It readily bonds with other elements to complete its outer electron shell, often forming stable compounds such as water (H2O) and carbon dioxide (CO2).
When it comes to the role of oxygen in compounds, it is essential to understand its valence electrons and how it forms different types of compounds. Oxygen, with an atomic number of 8, has six valence electrons in its outermost energy level. These valence electrons play a crucial role in determining the chemical behavior of oxygen and its ability to bond with other elements.
Oxides Formation
Oxygen readily forms oxides by combining with other elements. Oxides are compounds that contain oxygen and one or more additional elements. The formation of oxides occurs through various chemical reactions, such as oxidation and combustion. – Oxides can be classified into different types based on their chemical properties.
Some common types of oxides include: – Basic oxides: These oxides react with acids to form salts and water. They typically have a metal element combined with oxygen, such as calcium oxide (CaO) or magnesium oxide (MgO). – Acidic oxides: These oxides react with bases to form salts and water. They often contain a non-metal element combined with oxygen, like carbon dioxide (CO2) or sulfur dioxide (SO2). – Amphoteric oxides: These oxides exhibit both acidic and basic properties. They can react with both acids and bases to form salts and water. One example is aluminum oxide (Al2O3).
Organic Compounds And Oxygen
Oxygen also plays a vital role in organic compounds, which are compounds that contain carbon atoms. In organic molecules, oxygen can be found in various functional groups, such as alcohols, ethers, and carbonyl compounds. – Alcohols: Alcohols are organic compounds that contain a hydroxyl (-OH) group. Oxygen is bonded to a carbon atom, which is also bonded to hydrogen atoms. Ethanol (C2H5OH) is an example of an alcohol. – Ethers: Ethers have an oxygen atom bonded to two carbon atoms. They are commonly used as solvents and anesthetics.
An example of an ether is dimethyl ether (CH3OCH3). – Carbonyl compounds: Carbonyl compounds contain a carbon-oxygen double bond (C=O). They include aldehydes and ketones. Acetone (CH3COCH3) is a well-known ketone. In conclusion, oxygen’s valence electrons determine its chemical behavior and ability to form various compounds. It readily combines with other elements to form oxides and plays a crucial role in organic compounds. Understanding the role of oxygen in compounds is essential for studying chemical reactions and the properties of different substances.
Valence Variations: Exceptions And Excitement
Oxygen Anions And Cations
Oxygen can form anions by gaining extra electrons and cations by losing electrons.
Unusual Oxidation States
Oxygen exhibits uncommon oxidation states, such as -1 in peroxides and -2 in superoxides.
Practical Implications Of Oxygen’s Valency
Oxygen, with 6 valence electrons, plays a crucial role in various applications.
Biological Significance
Oxygen’s valency is vital for cellular respiration and sustaining life forms.
Industrial Applications
Oxygen’s valency is key in steel production and medical applications.
Interactive Learning: Oxygen’s Valence Electrons
Oxygen has six valence electrons, which are the outermost electrons in its electron cloud. Interactive learning tools can help students understand the concept of valence electrons and their role in chemical bonding.
Laboratory Experiments
Discovering oxygen’s valence electrons through interactive learning is engaging and insightful. Let’s explore more!
Oxygen, a crucial element, has six valence electrons. Educational simulations and laboratory experiments are great ways to understand this concept better.
Credit: praxilabs.com
Frequently Asked Questions
How Many Valence Electrons Does Oxygen Have?
Oxygen has 6 valence electrons. Valence electrons are the electrons in the outermost shell of an atom, and they are involved in the formation of chemical bonds. Understanding the number of valence electrons in an atom helps predict its chemical behavior.
Why Are Valence Electrons Important In Chemistry?
Valence electrons determine an element’s reactivity and its ability to form chemical bonds with other elements. Understanding the number of valence electrons helps predict the type of bond an element can form, whether it’s ionic, covalent, or metallic.
How Do Valence Electrons Affect The Properties Of Oxygen?
The 6 valence electrons of oxygen enable it to form covalent bonds with other elements, contributing to its role in supporting life through respiration and various chemical reactions. The properties of oxygen, such as its reactivity and bonding behavior, are influenced by its valence electrons.
Conclusion
Understanding the number of valence electrons in oxygen is crucial for comprehending its chemical behavior. With six valence electrons, oxygen forms stable compounds and plays a vital role in various biological and industrial processes. By grasping this concept, you can gain a deeper insight into the properties and reactivity of oxygen.





